Clinical Evaluation Report for Europe and MEDDEV Expectations: Impact with New Medical Device Regulations (MDR) | Medical Events

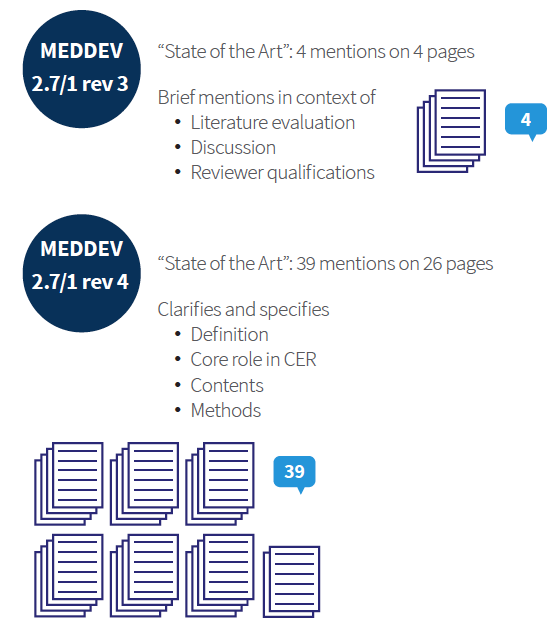
MEDDEV 2.7.1 Rev 4: New Requirements and Changes for Clinical Evaluation Reports (CER) - GMED Medical Device Certification