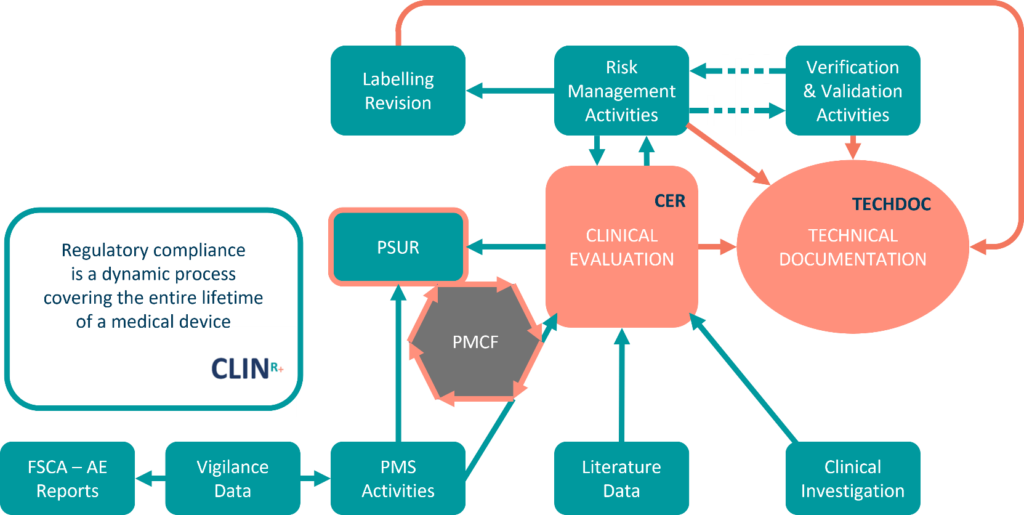
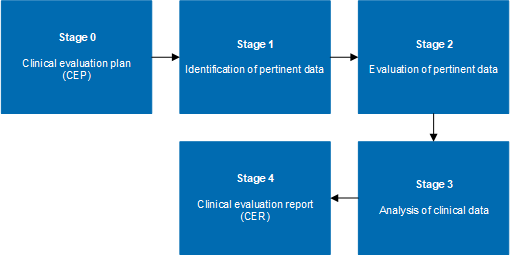
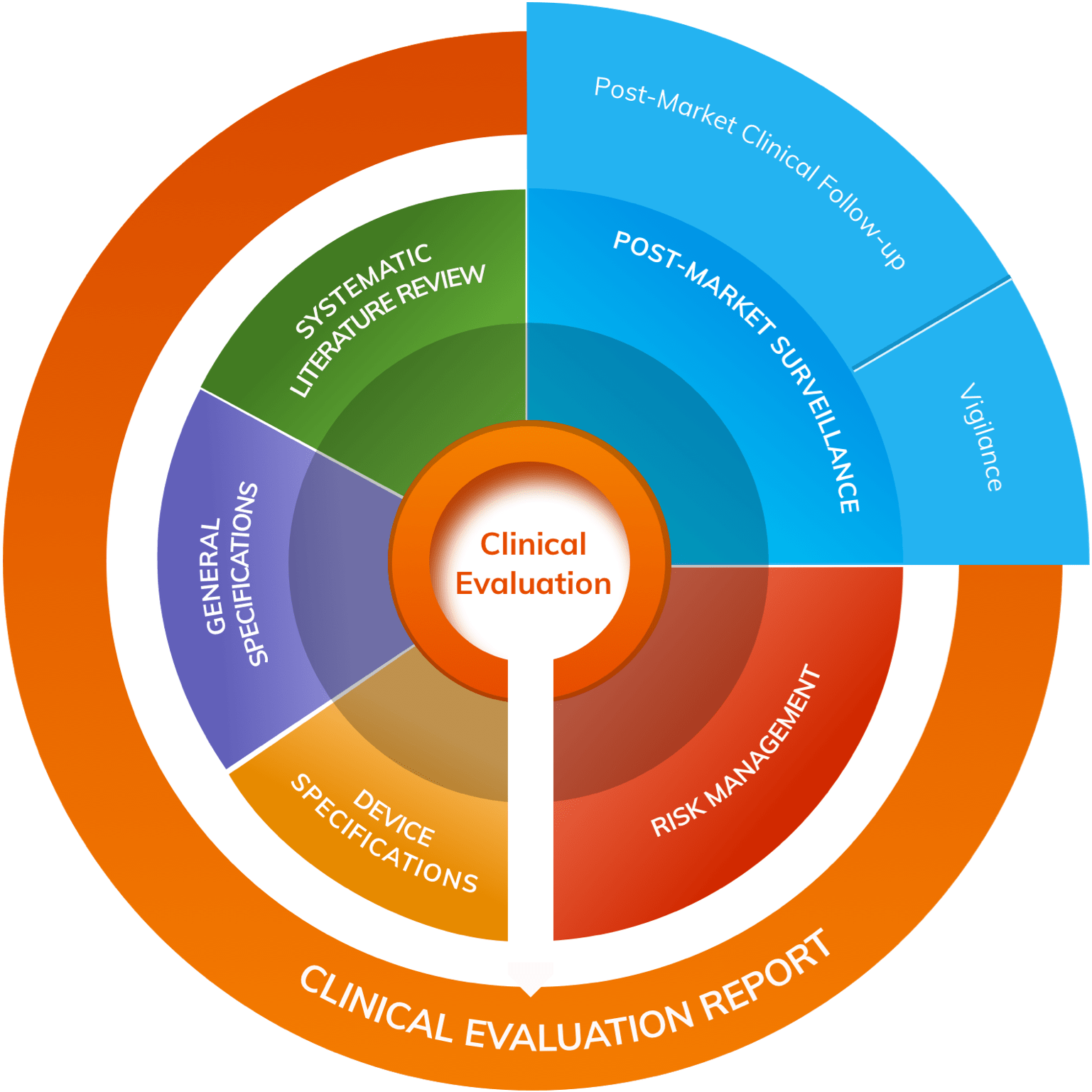
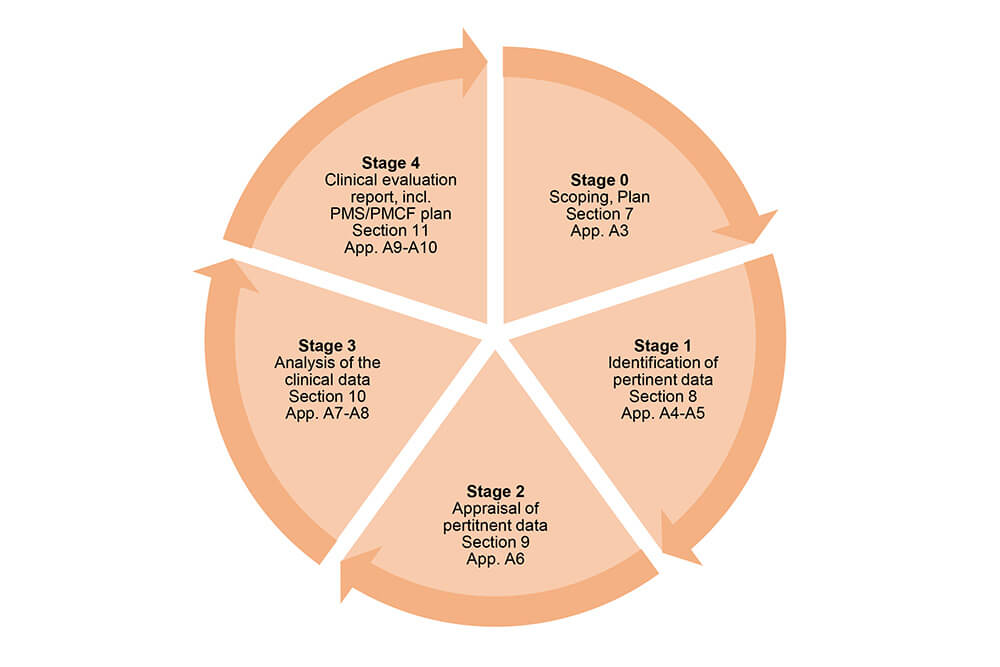
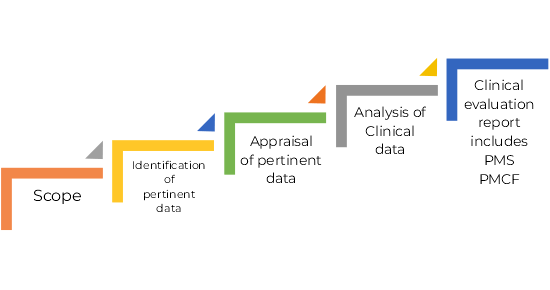
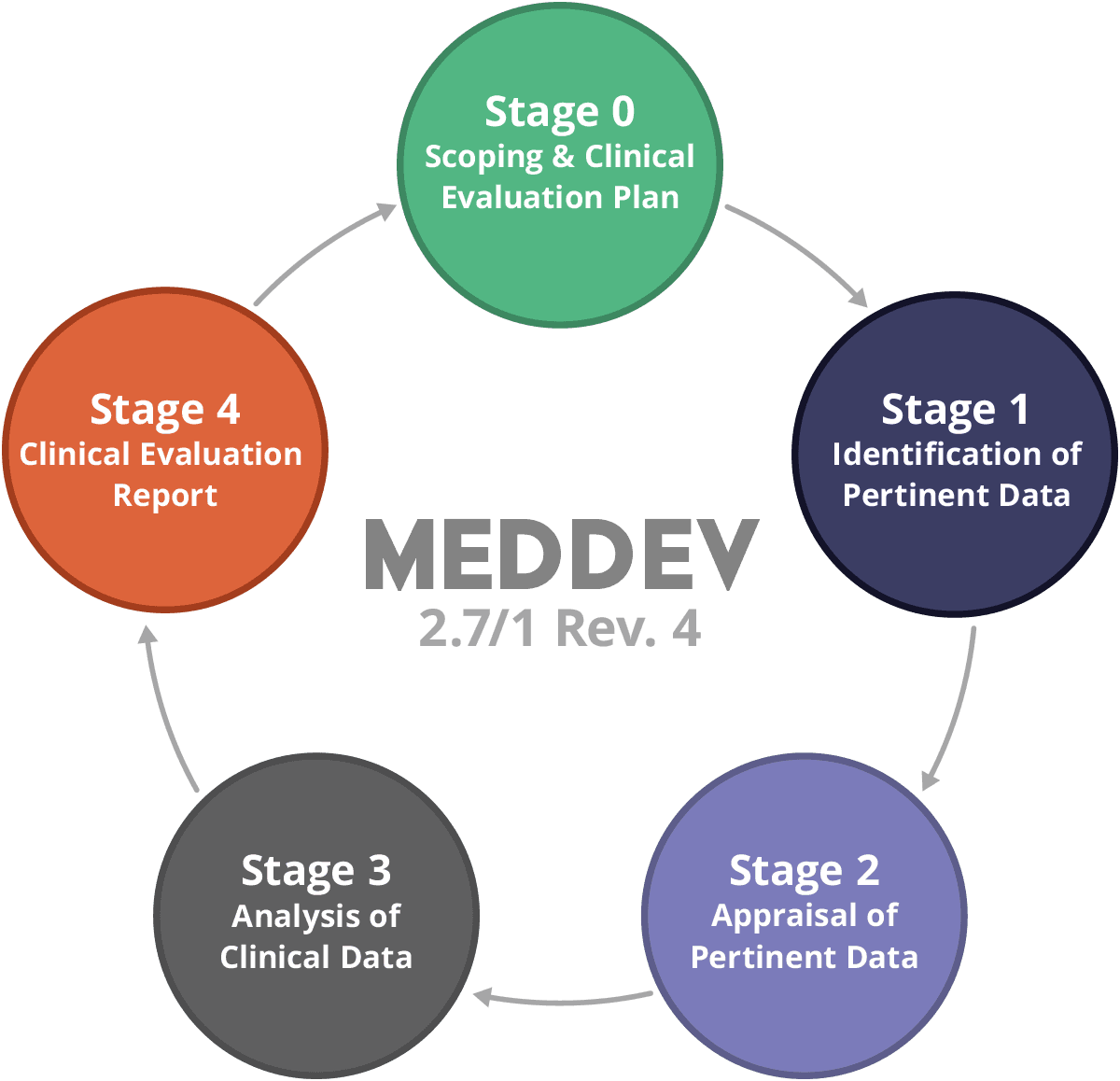
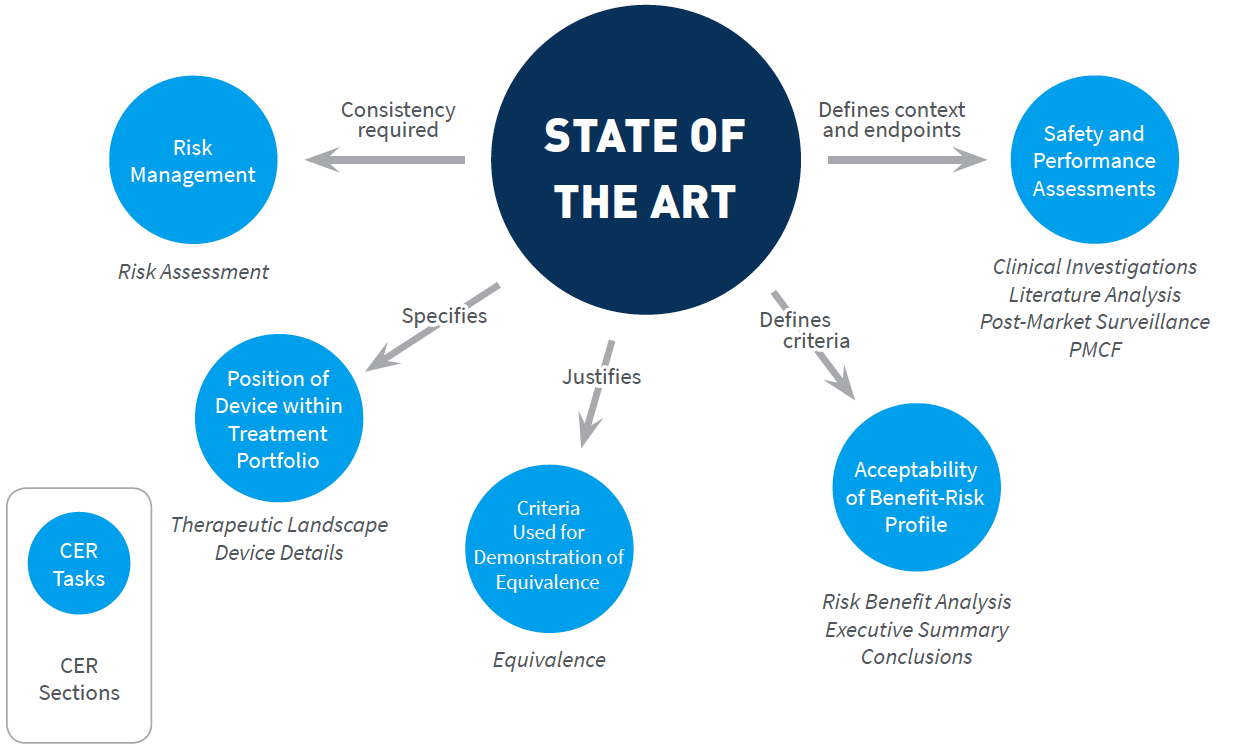
Medical Device Regulation 2017/745 Clinical Evaluation Report: Generalities · MDlaw – Information platform on European medical device regulations
White Paper Clinical Evaluation of Medical Devices: The Increasing Responsibilities of Clinical Evaluators