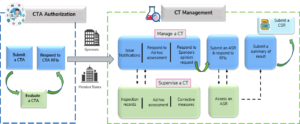
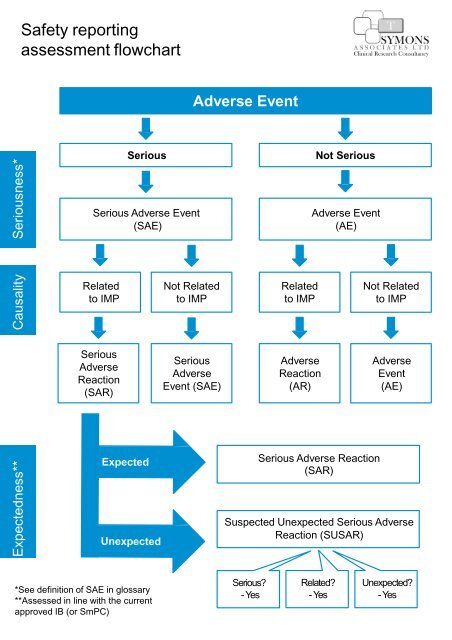
Safety Reporting Overload in Clinical Trials: FDA and Site Perspectives on Overreporting of Adverse Events | CenterWatch

European Medicines Agency on LinkedIn: Clinical Trials Information System: training and support - European…

SOLVED: In addition to serious adverse event reporting, what other reporting requirements exist for HGT trials? Quarterly enrollment statistics from all clinical trial sites Annual reports within 60 days of the anniversary