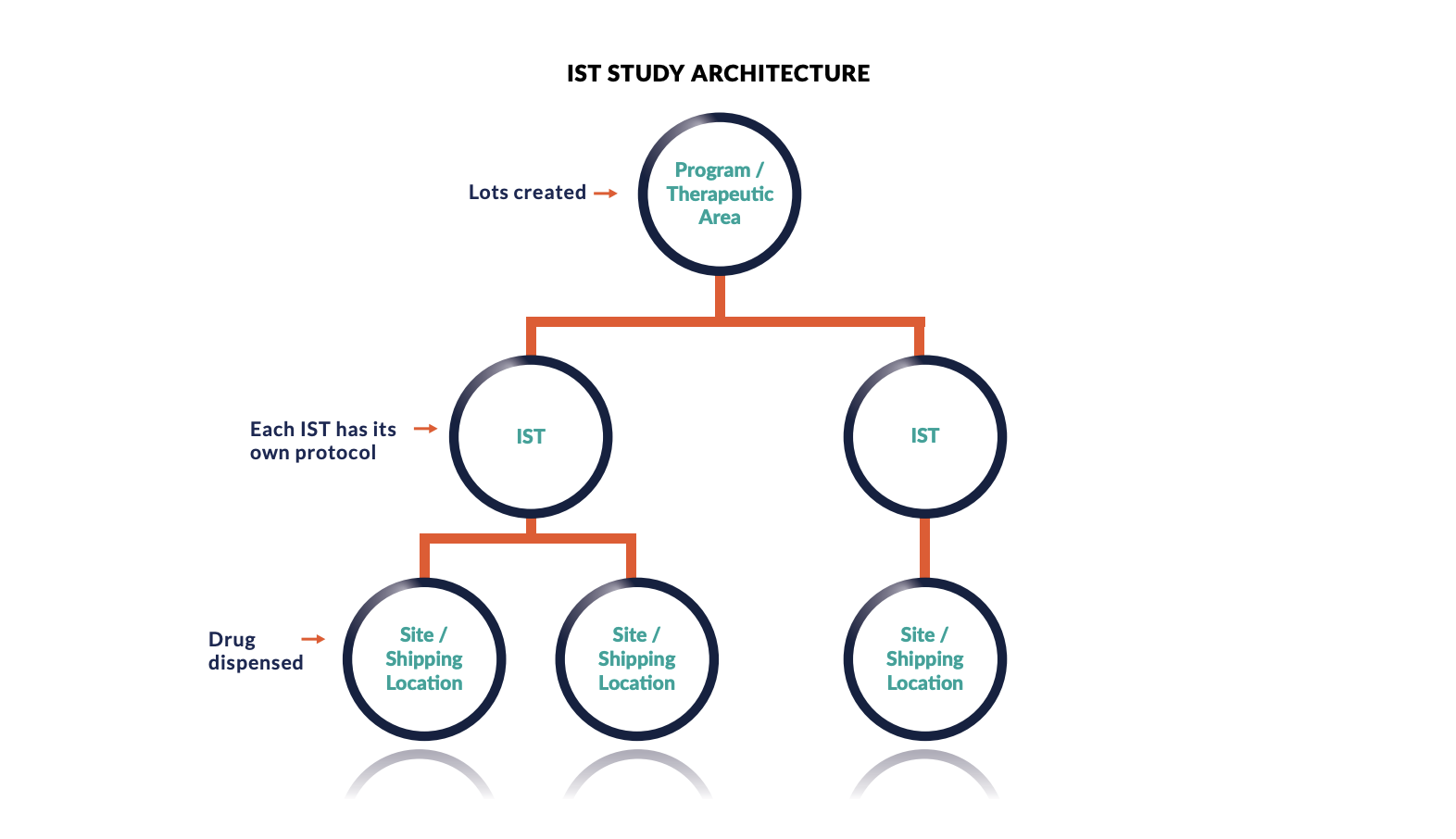
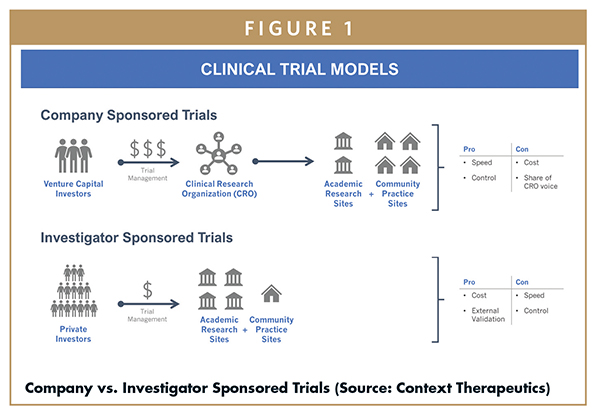
Investigator Initiated Studies, what are they and how can they be of value to your company? - Qserve CRO

A meta-research study revealed several challenges in obtaining placebos for investigator-initiated drug trials - ScienceDirect

Investigator Initiated Trials (IITs) Simplified: A Practical Guide for Clinical Trial Investigators to Conduct IITs: 9781540762894: Medicine & Health Science Books @ Amazon.com