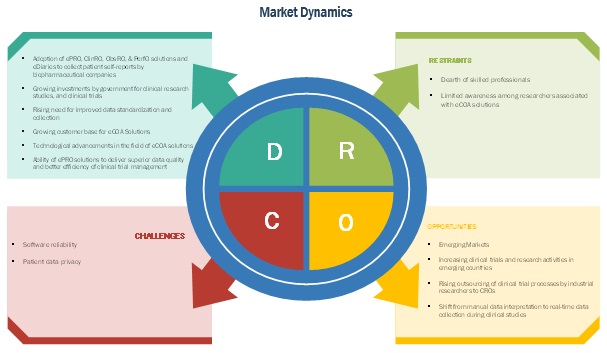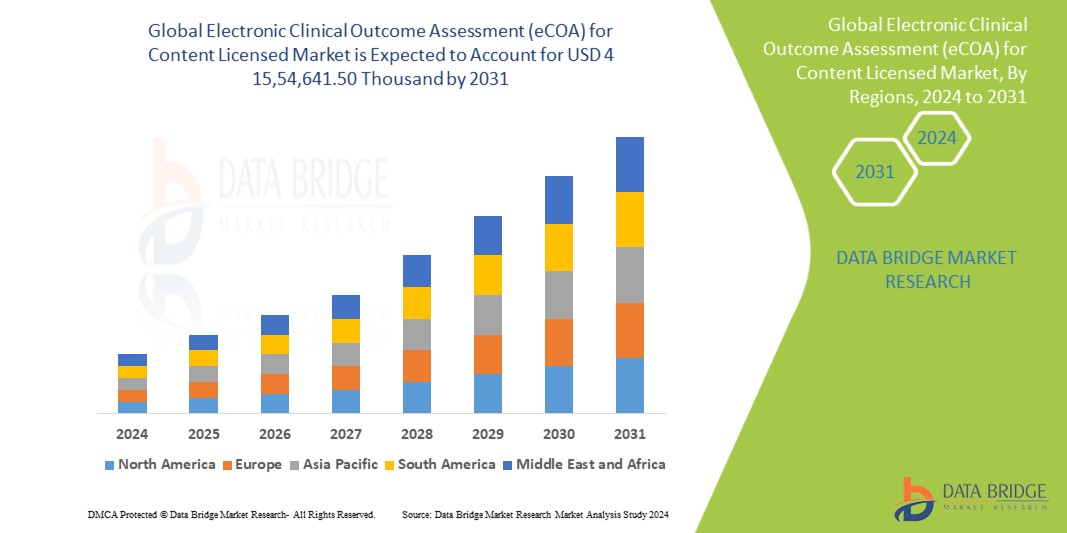
Electronic Clinical Outcome Assessment (eCOA) for Content Licensed Market – Global Industry Trends and Forecast to 2028 | Data Bridge Market Research
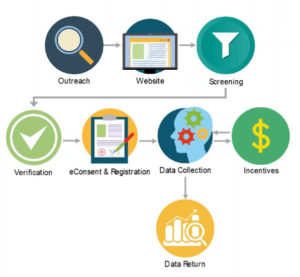
Three Times the Charm—Transforming Patient Centricity with eConsent, eCOA, and Patient Engagement - ACRP
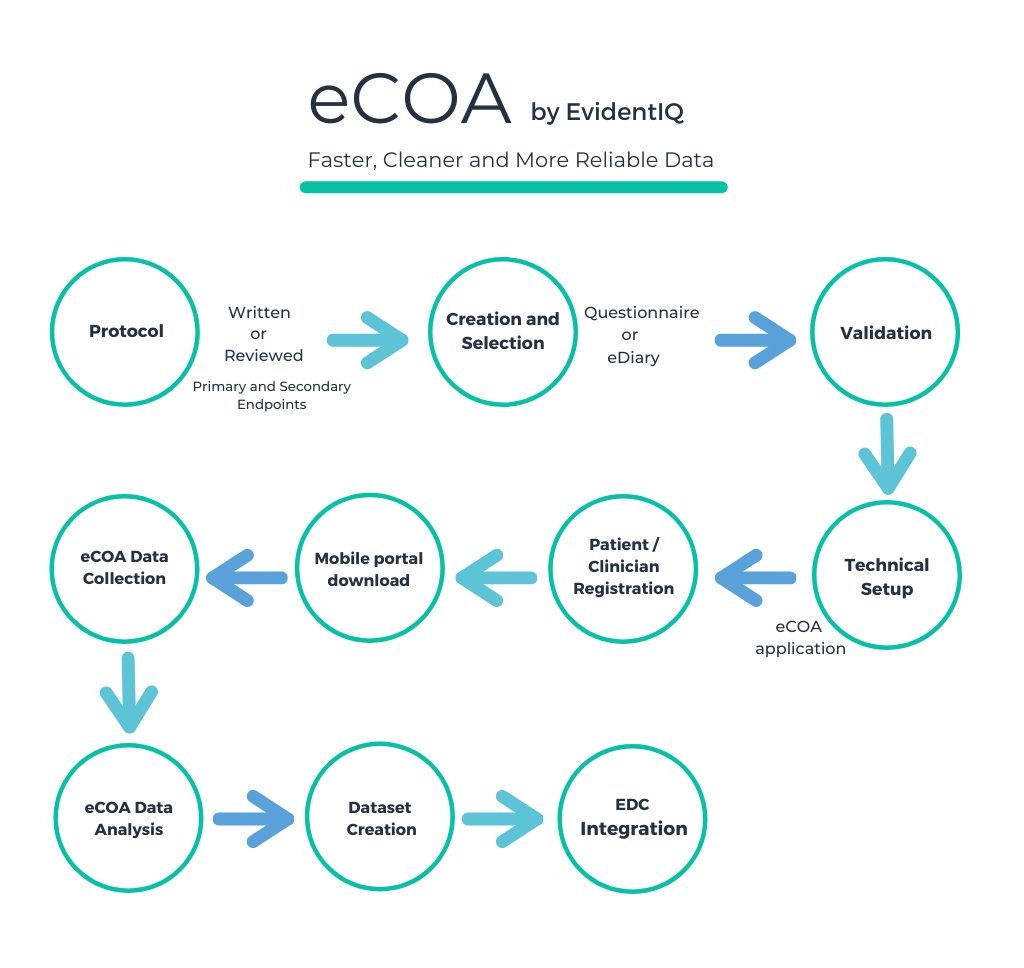
EvidentIQ group to launch new eCOA and eFeasibility offerings combining scientific services with a comprehensive software suite usable on any device, EvidentIQ Group GmbH, Press release - PresseBox
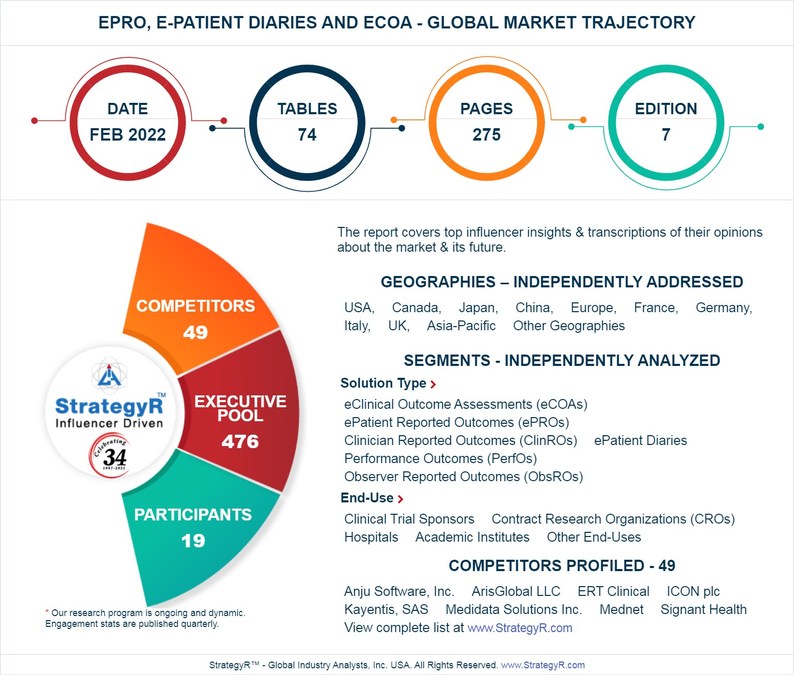
Valued to be $2.8 Billion by 2026, ePRO, E-Patient Diaries and eCOA Slated for Robust Growth Worldwide

Keep eCOA off the critical path of clinical trial startup - Clinical Research Services - ICON Webinar Channel
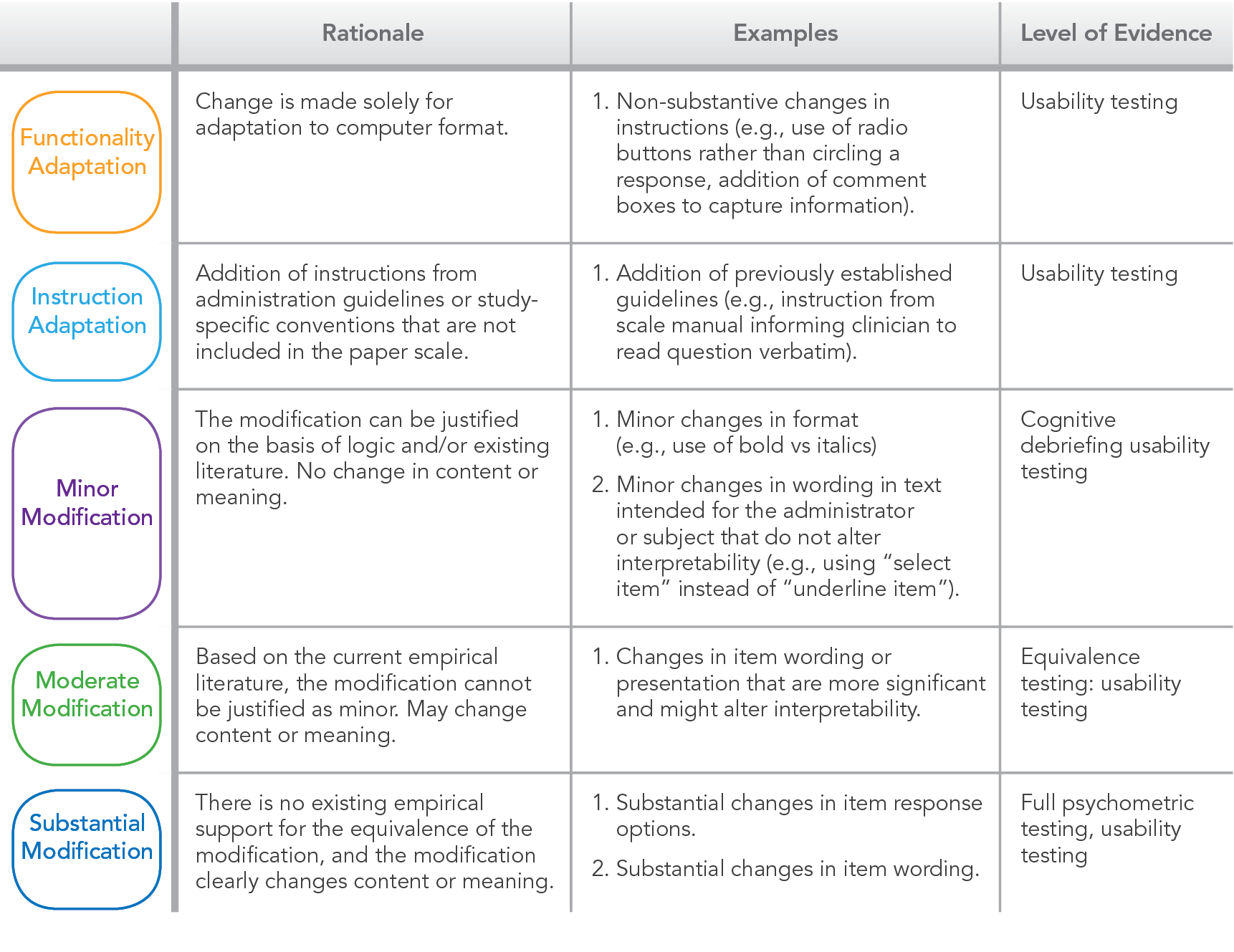
White Paper: Surprises You Don't Want When Adopting eCOAs for Use in Clinical Trials: Cautions for Decision Making and Planning - Evidera